- Your cart is empty
- Continue Shopping
Testing Your Runoff
Testing Your Runoff
Testing PPM’s and Elements: Why should I care?
PPM stands for “parts per million” and is a unit of concentration. For example: let’s say there are 3 lbs of cannabis compared to 1,000,000 lbs of water, we would say the concentration of cannabis is 3 ppm. Another unit of measure, ppb, is similar in principle but stands for “parts per billion.” When I am referring to PPM’s, I am referring to the Total Dissolved Solids (TDS), ot the total amount of nutrients present. Another measurement that people use is Electrical Conductivity (EC) – the working principle is the same. The more nutrients you have in your water (a higher concentration, more ppm’s), the higher your EC is because the nutrients are ionic solids.
When we are measuring PPM’s (usually with the same instrument that measures pH), we are analyzing the total amount of salt/nutrients in the water. This is important because we must make sure that we are adding the right amount of nutrients every feed. Let’s say you measure on Tuesday, and you get 1,500 ppm. You measure on Wednesday using the same feed and get 2,200 ppm in your reservoir. This can help you to conclude that too many nutrients were added one day vs. the other.
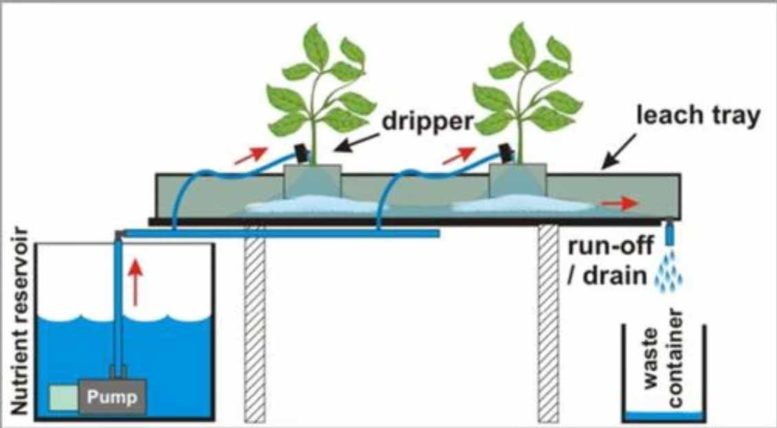
But this isn’t the only reason to measure PPM’s! Testing your runoff (the water stream beyond the root zone) provides useful pieces of information that can make sure your plants are performing the best that they can.
How to Measure Your Nutrient Concentration
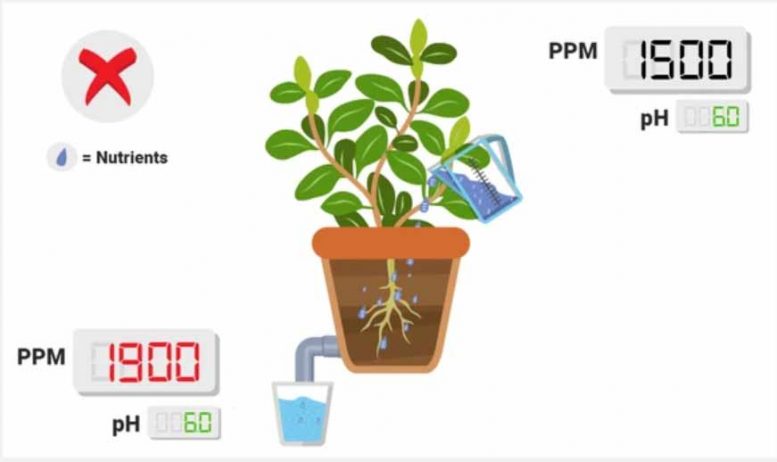
Every time you take a measurement, whether it be pH, ppm, or something else, you are conducting a science experiment. Like all science experiments, you must make sure they are done properly. I strongly recommend measuring two things in your water stream: ppm’s and pH; both must be measured in your reservoir and in your runoff. By measuring at a minimum of these two places, you can understand how your plants are behaving and potentially taking nutrients. Also – if you intend to get nutrient elementals, you must make sure to get the reservoir measurement as well as the runoff.
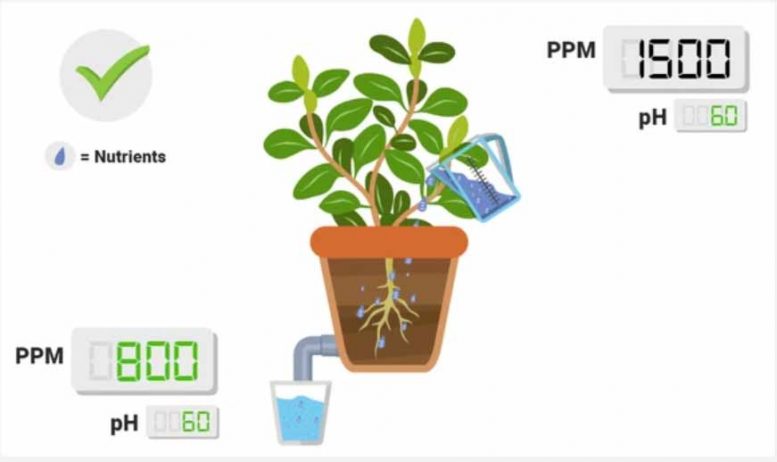
PPM lower in runoff than in reservoir .
This is a good thing! This implies that your plants are taking up nutrients, thus lowering the overall ionic concentration! If you monitor this routinely and you see a standard drop (e.g., 1600 in, 800 out, 6 days a week), that implies that your plants are experiencing roughly the same conditions on a daily level. Keep in mind: if your PPM’s are super low in the runoff, consider upping your nutrient treat rates slowly.
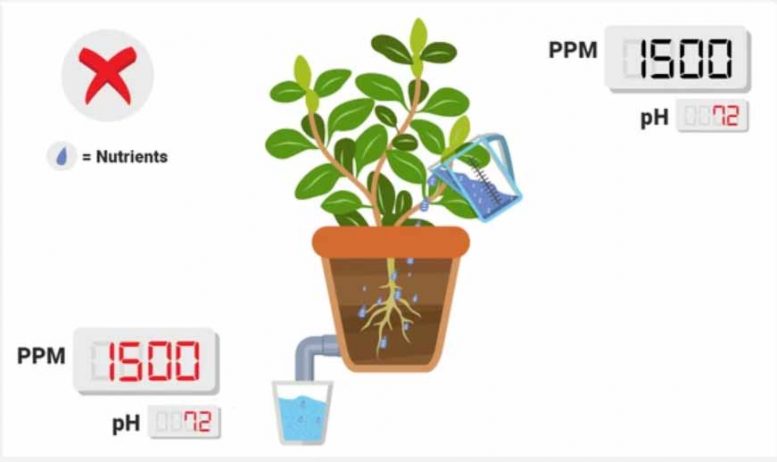
No PPM change between runoff and reservoir (or lower than normal drop).
This implies that your plants are not properly taking up the nutrients in the reservoir . One reason for this may be due to pH; certain ions (such as nitrate or phosphate) can only be taken up by the plant in certain ranges.
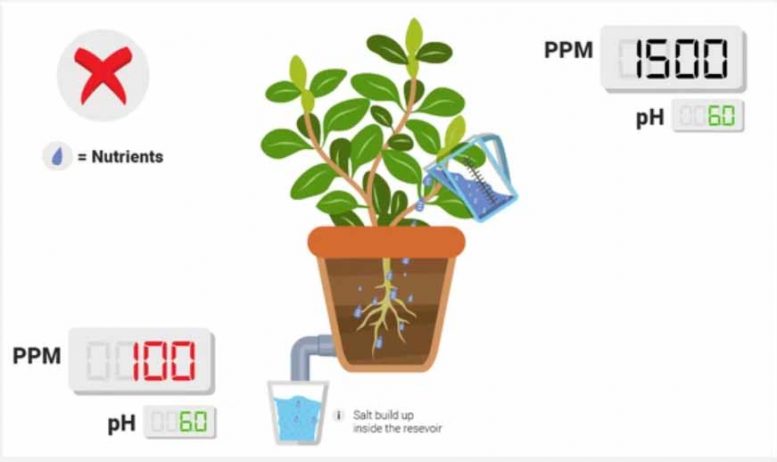
PPM is lower in reservoir than it usually is.
Testing Your Runoff
PPM Reading and what the mean
Check for a white precipitate (a solid) in your reservoir . One reason, if you’re sure you added the right amount of nutrients, that the ppm’s might be lower is due to nutrient lockout: not all the ions in your reservoir get along; they may react with one another and form a solid that is not soluble in water. One of the most classic culprits is calcium phosphate (from Ca2+ and PO43-). You can help avoid this by practicing proper mixing techniques.
PPM is higher in your runoff than reservoir .
Although rare, this indicates that there was some sort of salt buildup at the root zone. This buildup persists for a while, and then slowly dissolves back into the runoff, resulting in a higher PPM. The most common salts that are present in the root zone that are not water soluble are calcium-type salts (calcium phosphate, calcium carbonate, calcium hydroxide). Use a line cleaner (or if you’re at the end of the run and the rinse won’t hit the plants, try dilute muriatic acid) to help remove the salt buildup. Copious flushing with water will help too; it’s just slower.
Yellowing in the plants.
Look at the elemental values in your runoff vs. your reservoir . First, ensure that your nitrates (and total N), phosphates, and potassium ions are decreasing between your reservoir and runoff. This shows that the yellowing is not due to a macronutrient deficiency. Continue looking at the other elements to potentially find which element needs to be fortified. Again, if your PPM is not lower in your runoff than in your reservoir , there are some issues with your pH that may also be leading to the yellowing.
pH increase/decrease between reservoir and runoff.
A little variance is normal, but a large change is known as PH Drift. Keep an eye out for nutrient lockout, as this is one of the main reasons for a significant pH drift. It’s a good idea to monitor this because if your pH is drifting, certain nutrients may not be available to your plants as the pH drifts outside the uptake able range.